
RBGPF
0.1000


2 years after treatment, GelrinC-treated patients demonstrated layered cartilage architecture similar to native hyaline cartilage - widely regarded as the gold standard for durable joint function
This rare outcome in cartilage repair suggests true biological cartilage regeneration, not merely defect filling
Unlike traditional scaffolds, GelrinC limits fibrotic tissue overgrowth, supporting smoother joint motion and preserving natural biomechanics
HERZLIYA, IL / ACCESS Newswire / January 20, 2026 / Regentis Biomaterials Ltd., ("Regentis" or the "Company") (NYSE American:RGNT), a regenerative medicine company focused on innovative tissue repair solutions, today announced new long-term imaging data from its successfully completed European clinical trial of GelrinC®, demonstrating that regenerated cartilage exhibits internal structural organization closely resembling healthy, native hyaline cartilage.
The data, published in the peer-reviewed journal Cartilage, are based on an advanced MRI analysis of patients treated with GelrinC® for focal cartilage defects in the knee. MRI findings revealed progressive improvement in tissue organization over time, indicating continued maturation of the regenerated cartilage well beyond implantation. By 24 months, the repaired tissue exhibited a layered architecture comparable to native hyaline cartilage, which is widely regarded as the gold standard for long-term joint durability and function. This degree of structural organization suggests that GelrinC® supports the formation of cartilage with true biological quality, rather than fibrotic or scar-like repair tissue.
The analysis was conducted by Prof. Siegfried Trattnig of Vienna University and his colleagues, global leaders in cartilage MRI imaging, using validated methodologies accepted by both U.S. FDA and Europe's EMA regulators, further strengthening the translational and regulatory relevance of the findings.
"This data shows that GelrinC® helps regenerate cartilage that mirrors the structure of healthy, native tissue, going far beyond simply filling a defect," said Dr. Ehud Geller, Executive Chairman of Regentis. "These findings reinforce GelrinC®'s potential to deliver authentic, long-lasting cartilage regeneration and support our advancing Phase III U.S. FDA study and our commercialization efforts in Europe where GelrinC® has CE Mark approval."
In native cartilage, distinct layers are characterized by different collagen types-those associated with healthy hyaline cartilage and those linked to fibrotic repair. Remarkably, cartilage regenerated following GelrinC® treatment exhibited the same collagen-related layered pattern, indicating that the implant creates a biological environment conducive to authentic cartilage restoration.
GelrinC®'s injectable implant molds precisely to the cartilage defect, forming a seamless interface with surrounding tissue-an essential factor for long-term integration and mechanical stability. Unlike traditional scaffolds, GelrinC® has been shown to limit fibrotic tissue overgrowth, helping preserve smooth joint motion and natural biomechanics. Its unique surface chemistry and structural design are engineered to guide cellular organization, shaping not only healing but the quality and function of the regenerated cartilage.
Cartilage Regeneration with GelrinC®
These sequential MRI images illustrate the gradual regeneration and maturation of cartilage following GelrinC® treatment.
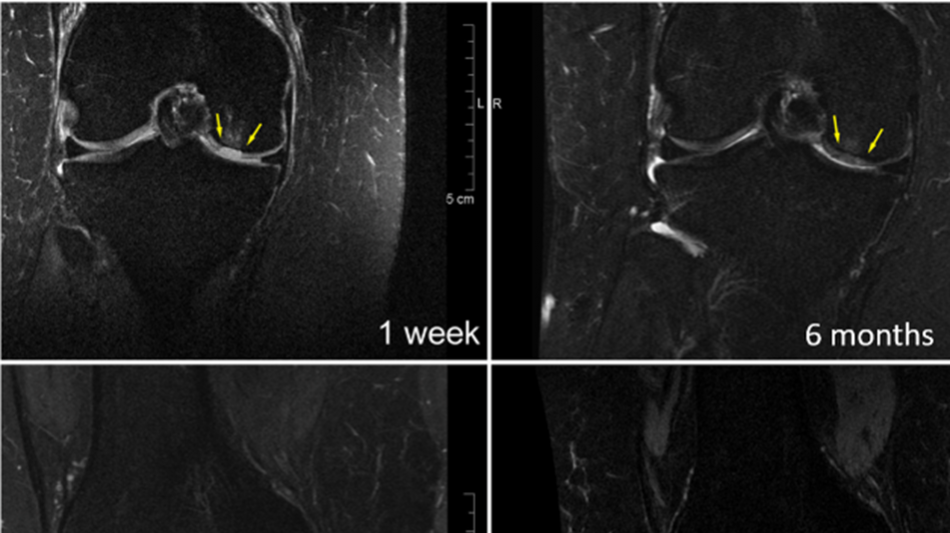
One week after treatment, the defect area is clearly visible. Over time, the images demonstrate progressive tissue formation and structural organization. By 12 months, the regenerated cartilage shows substantial improvement, and by 24 months, the defect is filled with well-organized cartilage tissue that closely resembles native cartilage in structure and quality, as reflected by the high MOCART score.
This slow and continuous maturation process suggests that GelrinC® supports durable cartilage regeneration, with tissue quality that continues to improve well beyond the initial healing phase.
About GelrinC®
Regentis' lead product, GelrinC®, is a cell-free, off-the-shelf hydrogel synchronized erosion and resorbable implant for the treatment of painful injuries to focal articular knee cartilage. As an innovative regenerative medical product, GelrinC® offers an unprecedented solution that gives surgeons and payers an off-the-shelf, ready to use, simple to perform, reliable, and cost-effective procedure that provides patients with a single, 10-minute procedure, faster recovery, sustained pain relief, and functional improvement for more than 4 years, based on clinical study results to date. No effective off-the-shelf, ready to use treatment for focal knee cartilage defects is currently available on the market. GelrinC® has CE Mark approval in the European Union and is now being evaluated in a pivotal U.S. Food and Drug Administration (FDA) study, which has completed over 50% enrollment.
About Regentis Biomaterials
Regentis Biomaterials Ltd is a regenerative medicine company dedicated to developing innovative tissue repair solutions that restore health and enhance quality of life. With an initial focus on orthopedic treatments, Regentis' Gelrin platform technology, based on synchronized, degradable hydrogel implants, regenerates damaged or diseased tissue including inflamed cartilage and bone. Regentis' lead product GelrinC®, is a cell-free, off-the-shelf hydrogel that is eroded and resorbed in the knee, allowing the surrounding cells to regenerate the cartilage in a controlled and synchronous process. GelrinC® aims to address a market of approximately 470,000 cases for cartilage knee repair annually in the U.S. where no off-the-shelf treatment is available.
Forward Looking Statements
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will" "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words, and include beliefs regarding Regentis' market positioning. Forward-looking statements are based on Regentis' current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. Factors that may affect future results and may cause these forward-looking statements to be inaccurate include, without limitation: the ability of our clinical trials to demonstrate safety and efficacy of GelrinC or any future product candidate, and other positive results; the timing and focus of our preclinical studies and clinical trials, and the reporting of data from those studies and trials; the size of the market opportunity for of GelrinC or any future product candidate, including our estimates of the number of patients who suffer from the diseases we are targeting; our ability to accurately identify demand for product candidates; the success of competing therapies that are or may become available; the beneficial characteristics, safety, efficacy and therapeutic effects of our product candidates; our ability to obtain FDA approval for of GelrinC or any future product candidate and obtain and maintain regulatory approval; our ability to obtain market acceptance of of GelrinC or any future product candidate from the medical community and third-party payors; our plans relating to the further development of GelrinC or any future product candidate, including additional disease states or indications we may pursue; existing regulations and regulatory developments in the United States and other jurisdictions; our plans and ability to obtain or protect intellectual property rights, including extensions of patent terms where available and our ability to avoid infringing the intellectual property rights of others; the need to hire additional personnel and our ability to attract and retain such personnel; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; our dependence on third parties; our financial performance and our ability to repay our loans and debts; and our ability to negotiate favorable terms in any collaboration, licensing or other arrangements into which we may enter and perform our obligations under such collaborations. For a more detailed description of the risks and uncertainties affecting Regentis, reference is made to the Company's reports filed from time to time with the Securities and Exchange Commission ("SEC"), including, but not limited to, the risks detailed in the section titled "Risk Factors" in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Regentis undertakes no duty to update such information except as required under applicable law.
Contact:
SOURCE: Regentis Biomaterials Ltd.
View the original press release on ACCESS Newswire
X.Silva--TFWP