
RBGPF
2.2900


In a TNBC animal model with limited responsiveness to chemotherapy, Telomir-1 demonstrated statistically significant activity on both tumor growth and cancer cell spread.
MIAMI, FLORIDA / ACCESS Newswire / January 5, 2026 / Telomir Pharmaceuticals (NASDAQ:TELO) ("Telomir" or the "Company"), a preclinical-stage biotechnology company developing small-molecule therapeutics targeting epigenetic and metabolic drivers of diseases, today announced results from a completed efficacy study evaluating Telomir-1 (Zn-Telomir) in zebrafish tumor xenograft animal models of triple-negative breast cancer (TNBC). The study was conducted in collaboration with BioReperia using its ZTX® ONCOLEADS platform.
In one of these animal models, Telomir-1 demonstrated statistically significant reductions in primary tumor growth and in the spread of cancer cells beyond the primary tumor (metastatic dissemination) in an aggressive TNBC model with limited responsiveness to the chemotherapy agent paclitaxel. In a separate aggressive TNBC model where paclitaxel demonstrated antitumor activity, Telomir-1 produced a comparable reduction in primary tumor growth as a monotherapy, and the combination treatment with Telomir-1 and paclitaxel resulted in greater tumor growth inhibition than either agent alone.
To visually illustrate these findings, representative images from BT549 xenografts and the HCC1806 metastases model are shown below, highlighting both primary tumor size and cancer cell spread following treatment with Telomir-1.
Representative Images: Primary Tumor Growth (BT549 xenografts) and Cancer Cell Spread (HCC1806 Cells)

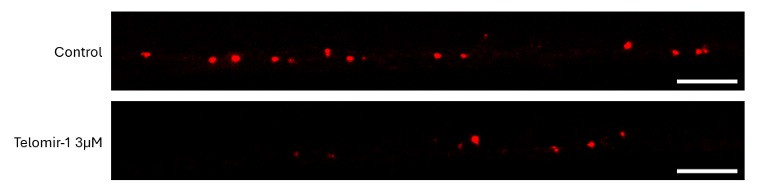
Representative zebrafish tumor xenograft images from the BT549 xenografts and HCC1806 triple-negative breast cancer models illustrating (A) primary BT549 xenografts tumor size and (B) cancer cell spread (metastatic dissemination) of HCC1806 cells. Images compare untreated control tumors with tumors treated with Telomir-1, demonstrating reduced primary tumor burden and reduced dissemination of cancer cells beyond the primary tumor site. Images are representative of quantified and statistically analyzed results reported in this study.
Study Design Overview
The study evaluated Telomir-1 administered alone and in combination with paclitaxel, a commonly used chemotherapy agent, on primary tumor growth and cancer cell spread in TNBC models. Human TNBC cells were implanted into zebrafish embryos and assessed over a three-day treatment period. Tumor size and cancer cell dissemination were quantified using fluorescent imaging and analyzed relative to vehicle-treated controls.
Three biologically distinct TNBC cell line-derived tumor models were evaluated to reflect the well-established heterogeneity of triple-negative breast cancer.
Why Triple-Negative Breast Cancer Is Not a Single Disease
Although triple-negative breast cancer is often discussed as a single clinical indication, it is now well recognized as a biologically heterogeneous disease, comprising multiple molecular subtypes with distinct drivers and treatment sensitivities.
Large genomic and transcriptomic studies have shown that TNBC can be divided into four to six major subtypes, including tumors that are initially chemotherapy-sensitive and others that are invasive and broadly treatment-resistant. As a result, no single therapy is expected to demonstrate uniform activity across all TNBC tumors, and variability in therapeutic response is an established feature of the disease.
From a drug-development perspective, this heterogeneity underscores the importance of treatment personalization, matching therapeutic mechanisms to tumor biology rather than pursuing unselected TNBC populations.
Tumor Model-Specific Results and Prevalence Context
BT-549 TNBC Model - Aggressive, Chemotherapy-Sensitive Subtype The BT-549 model represents an aggressive TNBC subtype with partial sensitivity to chemotherapy. In the current study, Telomir-1 and paclitaxel each produced statistically significant and broadly comparable reductions in primary tumor size when administered as monotherapies, with no statistically significant difference observed between the two agents. Combination treatment of low dose Telomir-1 and paclitaxel resulted in significantly greater tumor growth inhibition than either agent alone.
Based on published molecular subtype analyses, TNBC subtypes with biological features similar to BT-549 are estimated to represent approximately 30-40% of TNBC cases.
HCC1806 TNBC Model - Aggressive Subtype with Limited Chemotherapy Responsiveness The HCC1806 model represents an aggressive TNBC basal-subtype characterized by limited responsiveness to chemotherapy and a high propensity for cancer cell spread. In this model, paclitaxel did not produce statistically significant effects on primary tumor growth or on the spread of cancer cells beyond the primary tumor. In contrast, Telomir-1 treatment resulted in statistically significant reductions in primary tumor size at specific concentrations and statistically significant reductions in metastatic dissemination at an optimal concentration. Combination treatment further enhanced tumor growth inhibition compared to Telomir-1 monotherapy.
According to published molecular subtype analyses, TNBC subtypes with biological features similar to HCC1806, together with BT-549-like tumors, are estimated to account for approximately 40-60% of all TNBC cases.
MDA-MB-231 TNBC Model - Broadly Treatment-Resistant Subtype The MDA-MB-231 model represents a broadly treatment-resistant TNBC subtype is reported to exhibit tighter regulation of intracellular metal availability and strong intrinsic defense mechanisms. In this model, neither Telomir-1 nor the chemotherapy agent paclitaxel produced statistically significant effects on primary tumor growth or on the spread of cancer cells beyond the primary tumor.
TNBC subtypes with biological features similar to this model are estimated to represent approximately 15-25% of TNBC cases.
Mechanistic Interpretation: Iron, Copper, and Epigenetic Regulation
The differential responses observed across TNBC tumor models in this study are consistent with established differences in how these tumors regulate iron, copper, and epigenetically controlled gene activity.
TNBC models in which Telomir-1 demonstrated statistically significant effects are known, based on published literature, to rely on readily available intracellular iron and copper to support rapid growth and epigenetically regulated transcriptional programs. In these tumors, perturbation of metal availability is associated with measurable effects on tumor growth and cancer cell spread.
By contrast, the non-responsive TNBC model tightly controls and stores intracellular iron and copper and relies less on metal-regulated epigenetic flexibility. This biological profile is associated with reduced sensitivity to therapies that act through metal-dependent regulatory pathways and is consistent with the lack of response observed with both Telomir-1 and chemotherapy in this model.
Taken together, these findings indicate that Telomir-1 activity in this animal model is specific and dependent on underlying tumor biology and aligns with established differences among TNBC subtypes, rather than reflecting uniform or nonspecific anti-tumor effects.
Conclusion
· Telomir-1 demonstrated statistically significant tumor growth inhibition in aggressive TNBC models representing an estimated 40-60% of TNBC cases.
· Telomir-1 demonstrated statistically significant reduction in cancer cell spread in an aggressive TNBC model with limited chemotherapy responsiveness.
· In a broadly treatment-resistant TNBC subtype representing an estimated 15-25% of cases, neither Telomir-1 nor chemotherapy produced measurable effects, underscoring the biological specificity of response and providing important guidance for patient selection and clinical trial design.
· Combination treatment with paclitaxel enhanced tumor growth inhibition in models responsive to Telomir-1.
Importantly, the observed biology-driven activity and lack of effect in broadly treatment-resistant models provide valuable guidance for patient selection and trial design, which may improve the likelihood of observing clear clinical benefit in future studies.
Management Commentary
"These results demonstrate biologically consistent anti-tumor and anti-cancer spread activity in aggressive triple-negative breast cancer animal models, including tumors with limited responsiveness to standard chemotherapy, while also providing clear insight into the patient populations most likely to benefit in future clinical trials," said Erez Aminov, CEO of Telomir.
"From a scientific perspective, this study provides important validation that targeting iron- and copper-regulated epigenetic pathways can translate into measurable effects on both tumor growth and metastatic behavior in relevant TNBC subtypes," said Dr. Itzchak Angel, Chief Scientific Advisor at Telomir. "The alignment between mechanism and observed biology strengthens confidence as the program advances toward clinical development."
Next Steps
Telomir is advancing Telomir-1 through ongoing IND-enabling activities and is actively preparing the data package required to support a future Investigational New Drug (IND) submission. In parallel, the Company is refining indication selection and patient population strategies based on accumulated preclinical evidence to support regulatory readiness and informed clinical development planning.
About Telomir Pharmaceuticals
Telomir Pharmaceuticals (NASDAQ:TELO) is a preclinical biotechnology company developing small-molecule therapeutics designed to target the root epigenetic mechanisms underlying cancer, aging, and degenerative disease. The Company's lead candidate, Telomir-1, has demonstrated activity in preclinical studies involving modulation of DNA and histone methylation, restoration of redox balance, and normalization of cellular function.
Cautionary Note Regarding Forward-Looking Statements
This press release, statements of Telomir's management or advisors related thereto, and the statements contained in the news story linked in this release contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications, and the safety of Telomir-1.
Any forward-looking statements in this press release are based on Telomir's current expectations, estimates and projections only as of the date of this release. These and other risks concerning Telomir's programs and operations are described in additional detail in its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, which are on file with the SEC and available at www.sec.gov. Telomir explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Email: [email protected] Phone: (786) 396-6723
SOURCE: Telomir Pharmaceuticals, Inc
View the original press release on ACCESS Newswire
W.Knight--TFWP