
SCS
0.0200


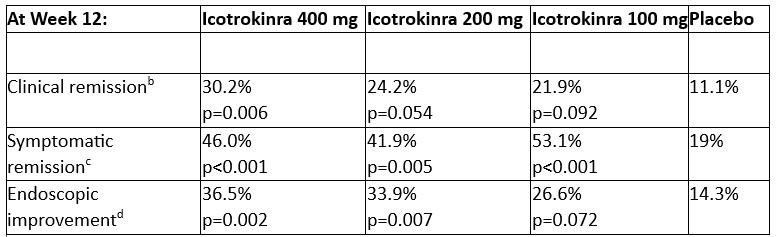
Icotrokinra met the primary endpoint of clinical response at all three doses, with 36.5% of patients treated achieving endoscopic improvement and 30.2% achieving clinical remission at the highest dose at Week 12 in the Phase 2b ANTHEM-UC study
These data support the promise of a first-in-class targeted oral peptide that selectively blocks the IL-23 receptor as a potential new option for people with moderately to severely active ulcerative colitis
Registrational Phase 3 study in ulcerative colitis and Phase 2b/3 study in Crohn's disease anticipated to begin patient enrollment in Q4 2025
NEWARK, CALIFORNIA / ACCESS Newswire / October 7, 2025 / Protagonist Therapeutics, Inc. ("Protagonist" or the "Company") today announced additional Week 12 results from the Phase 2b ANTHEM-UC study of icotrokinra, a first-in-class investigational targeted oral peptide that selectively blocks the IL-23 receptor, in adults with moderately to severely active ulcerative colitis (UC). The study met its primary endpoint, with all once-daily icotrokinra dose groups achieving clinical responsea at Week 12 and showing clinically meaningful improvements versus placebo across key secondary endpoints.1 These results underscore the potential of icotrokinra to deliver a valuable combination of significant therapeutic benefit and a favorable safety profile with once-daily oral dosing and are featured at United European Gastroenterology (UEG) Week 2025.
At Week 12, patients treated with 400 mg of icotrokinra once daily achieved a clinical response rate of 63.5% versus 27% for placebo (p1
Across multiple secondary endpoints, in the 400 mg icotrokinra group, significantly greater proportions of patients achieved clinical remission, symptomatic remission, and endoscopic improvement at Week 12 compared to placebo. Both the 200 mg and 100 mg once-daily dosing groups also showed meaningful improvements in these secondary endpoints relative to placebo. All icotrokinra doses demonstrated higher rates of symptomatic remission compared to placebo as early as Week 4.1
Similar proportions of participants reported adverse events and serious adverse events through Week 12 across all icotrokinra dose groups and the placebo group.1
Based on results from the Phase 2b ANTHEM-UC study, a Phase 3 trial in ulcerative colitis will be initiated. Icotrokinra is also being studied in the pivotal Phase 3 ICONIC program in moderate-to-severe plaque psoriasis and the ICONIC-PSA 1 and ICONIC-PSA 2 studies in active psoriatic arthritis. A New Drug Application (NDA) was submitted to the U.S. Food and Drug Administration (FDA) in July 2025 seeking the first approval of icotrokinra for the treatment of adults and pediatric patients 12 years of age and older with moderate to severe plaque psoriasis.
"The vast body of compelling data from Phase 2b and Phase 3 studies, including the most recent results from this Phase 2b study in ulcerative colitis, supports the thesis of IL-23R targeted oral peptide icotrokinra as a potential treatment option in a range of inflammatory and immunological diseases," said Dinesh V. Patel, Ph.D., President and Chief Executive Officer at Protagonist. "We are pleased to see the recent initiation of the registrational Phase 3 study of icotrokinra in UC and a Phase 2b/3 study in Crohn's disease by our partner, along with the ongoing Phase 3 studies in psoriatic arthritis and the Phase 3 head-to-head results vs. ustekinumab in plaque psoriasis. We are also excited about the initiation of the Phase 1 study of our fully-owned oral IL-17 targeted oral peptide PN-881. Our goal is to deliver well-differentiated oral treatments for patients as we continue to advance our scientific and innovation leadership in the I&I field."
Editor's notes:
Clinical response is defined as a decrease from baseline in the modified Mayo score by greater than or equal to (>=) 30 percent (%) and >=2 points, with either a >=1-point decrease from baseline in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
Clinical remission was defined as a Mayo stool frequency subscore of 0 or 1, a Mayo rectal bleeding subscore of 0, and a Mayo endoscopic subscore of 0 or 1.
Symptomatic remission per Mayo score is defined as a stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0.
Endoscopic improvement was defined as an endoscopy subscore of 0 or 1.
About ANTHEM-UC
ANTHEM-UC (NCT06049017) is a Phase 2b multicenter, randomized, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of icotrokinra (JNJ-77242113, JNJ-2113) in patients with moderately to severely active ulcerative colitis who had an inadequate response or intolerance to conventional therapy (e.g., thiopurines or corticosteroids), prior biologics (TNF antagonists or vedolizumab) and/or ozanimod or approved JAK inhibitors. The study is evaluating three once-daily dosages of icotrokinra taken orally.2
About Ulcerative Colitis
Ulcerative colitis (UC) is a chronic disease of the large intestine, also known as the colon, in which the lining of the colon becomes inflamed and develops tiny open sores, or ulcers, that produce pus and mucus. It is the result of the immune system's overactive response. Symptoms vary but may typically include loose and more urgent bowel movements, rectal bleeding or bloody stool, persistent diarrhea, abdominal pain, loss of appetite, weight loss, and fatigue.3
About Protagonist
Protagonist Therapeutics is a discovery through late-stage development biopharmaceutical company. Two novel peptides derived from Protagonist's proprietary discovery platform are currently in advanced Phase 3 clinical development, with New Drug Application (NDA) for icotrokinra submitted to the FDA in July, and in the NDA submission for rusfertide expected by end of 2025. Icotrokinra (formerly, JNJ-2113), is a first-in-class investigational targeted oral peptide that selectively blocks the Interleukin-23 receptor ("IL-23R"), which is licensed to Janssen Biotech, Inc., a Johnson & Johnson company. Following icotrokinra's joint discovery by Protagonist and Johnson & Johnson scientists pursuant to the companies' IL-23R collaboration, Protagonist was primarily responsible for the development of icotrokinra through Phase 1, with Johnson & Johnson assuming responsibility for development in Phase 2 and beyond. Rusfertide, a mimetic of the natural hormone hepcidin, is currently in Phase 3 development for the rare blood disorder polycythemia vera (PV). Rusfertide is being co-developed and will be co-commercialized with Takeda Pharmaceuticals pursuant to a worldwide collaboration and license agreement entered in 2024 under which the Company remains primarily responsible for development through NDA filing. The Company also has a number of preclinical stage drug discovery programs addressing clinically and commercially validated targets, including IL-17 oral peptide antagonist PN-881, obesity triple agonist peptide PN-477, and the oral hepcidin program.
More information on Protagonist, its pipeline drug candidates, and clinical studies can be found on the Company's website at https://www.protagonist-inc.com/.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the potential benefits of icotrokinra and PN-881, and expectations regarding the icotrokinra and PN-881 development programs. In some cases, you can identify these statements by forward-looking words such as "anticipate," "believe," "may," "will," "expect," or the negative or plural of these words or similar expressions. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, our ability to develop and commercialize our product candidates, our ability to earn milestone payments under our collaboration agreements with Janssen and Takeda, our ability to use and expand our programs to build a pipeline of product candidates, our ability to obtain and maintain regulatory approval of our product candidates, our ability to operate in a competitive industry and compete successfully against competitors that have greater resources than we do, and our ability to obtain and adequately protect intellectual property rights for our product candidates. Additional information concerning these and other risk factors affecting our business can be found in our periodic filings with the Securities and Exchange Commission, including under the heading "Risk Factors" contained in our most recently filed periodic reports on Form 10-K and Form 10-Q filed with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition, and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements, whether as a result of new information, future events, or otherwise, after the date of this press release.
Investor Relations Contact
Corey Davis, Ph.D.
LifeSci Advisors
+1 212 915 2577
[email protected]
Media Contact
Virginia Amann, Founder/CEO
+1 833 500 0061 ext 1
ENTENTE Network of Companies
[email protected]
References:
1Abreu M., et al. Icotrokinra, a targeted oral peptide that selectively blocks IL-23 receptor activation, in moderately to severely active ulcerative colitis: week 12 results from the phase 2b, randomized, double-blind, placebo-controlled, treat-through, dose-ranging ANTHEMUC trial. Oral presentation OP206 at United European Gastroenterology Week (UEGW) 2025. October 2025.
2Clinicaltrials.gov. A Study of JNJ-77242113 in Participants With Moderately to Severely Active Ulcerative Colitis (ANTHEM-UC). Identifier NCT06049017. https://clinicaltrials.gov/study/NCT06049017?term=ANTHEM-UC&rank=1. Accessed February 2025.
3Crohn's & Colitis Foundation. What is ulcerative colitis? Available at: https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis. Accessed April 2024.
SOURCE: Protagonist Therapeutics
View the original press release on ACCESS Newswire
W.Lane--TFWP